Thierry Douki & Jean-Luc Ravanat Numerous compounds exhibit electrophilic properties and react with biomolecules through the formation of covalent and stable bonds. These modifications lead to deleterious cellular responses. They are involved in the effects of
pollution and many organic compounds. Electrophilic and alkylating drugs are also used to kill tumor cells. DNA is one of the cellular targets of electrophilic chemicals and the resulting damaged are known as
DNA adducts. Although the phosphodiester bonds can be affected, the vast majority of the DNA adducts involve the bases. The heterocyclic nitrogen atoms as well as the exocyclic amino and carbonyl groups are involved in the formation of adducts. Some of them result from the creation of carbon-carbon bonds. The chemical structure of the adduct depends on the nature of the electrophile species. Soft electrophiles react preferentially with the exocyclic amino groups while harder reagents add to the heterocyclic imine or the exocyclic carbonyls. This diversity in terms of chemical reactivity has major impact on the biological consequences of the adducts. Indeed, their mutational properties are different whether one part of a base is affected or the other. In addition, the four DNA bases are not equally affected by different electrophiles. The expertise of CIBEST in structure identification and analytical chemistry (
HPLC-MS/MS) allows us to precisely describe the distribution of the different types of DNA adducts.
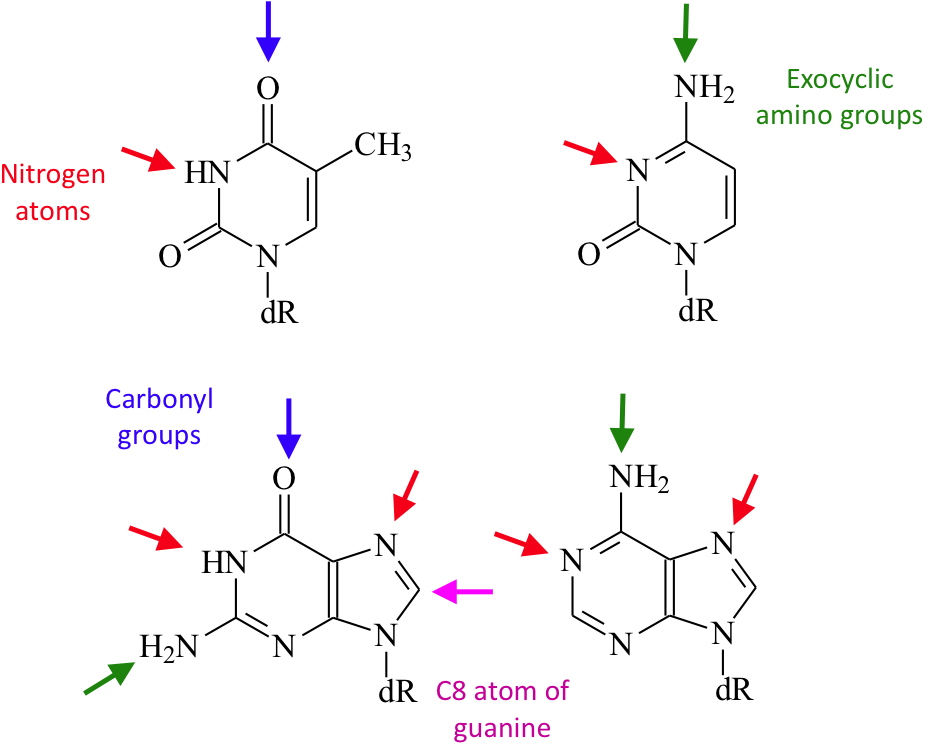
Reactive sites of DNA bases towards electrophilic species.
The DNA adducts-generating compounds can be classified in two types. Some chemicals are directly reactive with DNA while others require metabolic activation. In the last case, metabolites rather than the parent molecule are thus the damaging agent. This is typically true for polycyclic aromatic hydrocarbons (PAH) extensively studied at CIBEST. When entering cells, PAHs like benzo[a]pyrene are metabolized by numerous enzymes such as cytochrome P450 oxygenases. In the case of B[a]P, the most carcinogenic metabolite has been shown to be a diol epoxide that preferentially adds to the exocyclic amino groups of guanine. In the studies of the genotoxic action of PAHs, CIBEST combines the quantification of the expression of CYP genes by RT-PCR with HPLC-MS/MS measurement of the level of DNA adducts. Measurement of the activity of CYP450 provides additional information. This combined approach yielded relevant information on the effects of realistic mixtures in cultured hepatocytes or human skin explants. The results show that synergistic and inhibitory effects are frequent in
mixtures and difficult to predict. We also studied combination of PAH with sunlight. Again, modulation of metabolism by this physical agent drastically changed the extent of DNA damage.
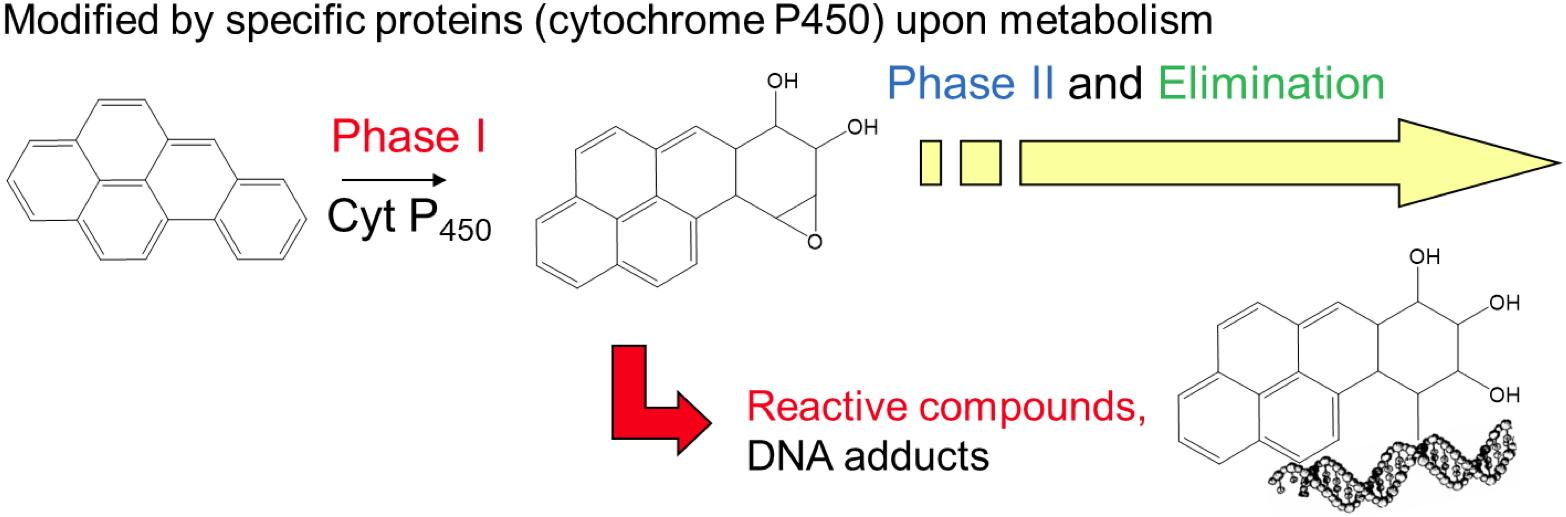
Benzo[a]pyrene is a typical genotoxic compound requiring metabolic activation.
Its diol-epoxide metabolite (BPDE) is extremely reactive towards DNA bases and produces BPDE-N2-Gua as the most frequent adduct.
Other genotoxic agents react without metabolic activation. A classic example is the alkylating agents, which add simple alkyl chains, or some of their substituted derivatives, to DNA bases. These reagents are mostly hard electrophile and react at position N7 on purine rings of guanine and adenine, and on the carbonyl group of guanine. Alkylation at position N7 induces a weakening of the bond between the base and sugar phosphate backbone. This facilitates the hydrolytic cleavage of the bond and the formation of an abasic site. Under some conditions, like those in the widely used Comet assay, this site is converted into a strand break. A typical alkylating agent is methyl methylsulfonate (MMS) often used as a positive control in genotoxicity assay.
Sulfur mustard and its derivatives, which are extensively studied at CIBEST, belong to this family. Other DNA damaging molecules do not necessitate metabolic activation. A typical example is cisplatin, an anti-tumor drug. Work is done at CIBEST on the formation and the
repair of its adducts in cells.
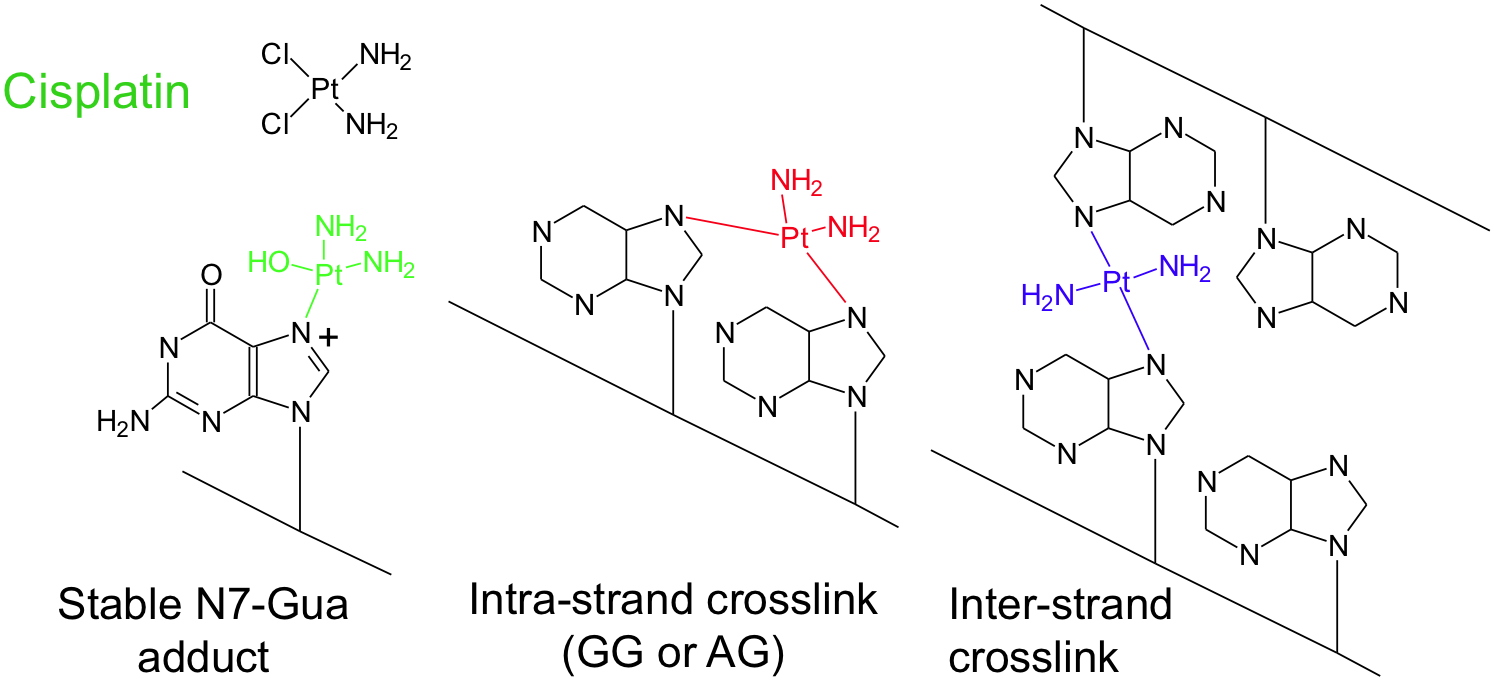
The antitumor drug cisplatin reacts in cells without metabolic activation.
Because it exhibits two reactive sites, it leads to the formation of monoadducts, of adducts between two adjacent bases or between bases of opposite strands.
The latter type of lesion, known as crosslinks is very toxic for the cell as it interfere with all the functions of DNA.